Genetics and mutations
DNA is a “building block for life”. But what exactly does this mean?
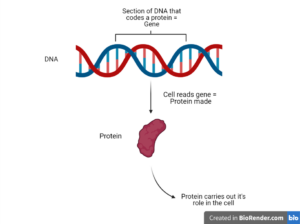
When a cell wants to make a new protein, it reads the specific DNA sequence which codes for that protein. This section of DNA sequence is a gene, with each gene coding for a different protein. In other words, the DNA is the programming for a cell, and the proteins are the pieces that carry out these instructions.
When mutations occur, the DNA sequence of a gene is changed. This means that when the cell then reads this altered code, an altered protein is produced. The programming has gone wrong. Many diseases, including cancer, are caused by gene mutations which affect the gene’s normal function. These genetic changes have been the focus of cancer research and drug development for decades.
However, the behaviour of your genes doesn’t just depend on its DNA sequence. It is also affected by epigenetic factors.