THE FUTURE OF RADIOTHERAPY
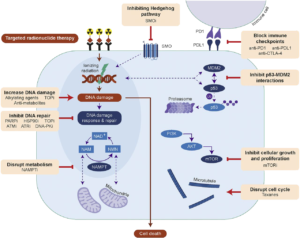
Combining TRT with other drugs gives better patient results than using either radiotherapy or the drug alone.
However, this is not the only benefit. Combination therapies may also mean lower doses of drugs and fewer treatment cycles may be required. This would help reduce the side effects prostate cancer patients experience, providing people with a better quality of life. Side effects not lifelong. Several drugs have shown promising early results and are now in clinical trials, with many more lined up for the future. Growing numbers of triple-combination therapies are also being tried out. Treatment outcomes are weighed up against any extra side-effects or impacts on patient quality of life.
Importantly, the review makes clear that more work into understanding exactly how TRT works, and how it interacts with the drugs it is being applied with. This is essential for effective and safe TRT combination therapies. It will also help us to stratify patients and tailor their treatment precisely to their cancer, helping more men survive prostate cancer, and with fewer side effects.